An interview with Professor Carolina Sales Vieira, INCT Hormona investigator at the University of São Paulo, Ribeirão Preto
Posted August 13, 2019
Professor Vieira, the lead author of the study “Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: a randomized controlled trial”, recently published in Contraception, discusses the evidence obtained by this study and other information of public health interest which has been acquired by INCT Hormona over the last decade. Enjoy!

A recent study which assessed the satisfaction of postpartum with a contraceptive implant (placed under the skin of the upper arm) found that there is no harm to women’s health associated with placing the implant immediately after delivery. What are women’s concerns about this method, and what evidence have you found to answer their questions?
Carolina Sales: When starting a hormonal contraceptive shortly after delivery, before hospital discharge, women are concerned about three things:
The first point is breastfeeding. Women want to know if the hormone released by the implant has any effect on their ability to breastfeed and whether it will change their milk volume. We addressed this concern in two pioneering studies in 2015 and 2017. The first study (2015) showed that the amount of milk produced by women is not reduced by an implant when it is placed within 48 hours after delivery. The second study (2017) showed that the rate of exclusive breastfeeding did not change in the first 6 months when we compared women who had the implant placed in the first 48 hours postpartum with those who had it placed after the conventional 6-week postpartum period. Thus, the timing of implant placement does not alter the amount of milk produced by breastfeeding women.
The second point concerns the effect of the hormone released by the implant on the baby, that is, women want to know if the hormone released by the implant will impair the child’s growth. In the 2017 study, we followed 100 babies and their mothers for a year: half of women had the implant placed within 48 hours of delivery, and half had the implant placed 6 weeks postpartum, which is the standard of care. We tracked the weight, height, head circumference, and arm circumference of all babies, and found no difference between the two groups. Thus, we can plainly state that the timing of implant placement does not affect the growth of babies breastfed by women who received a contraceptive implant.
The third question concerns the effect of the hormone released by the implant on the woman’s own health. Women want to know if having the implant placed just after delivery can harm them. We answered this question in our 2012 and 2019 studies. The greatest fear associated with using hormonal contraception in the first 6 weeks after giving birth is an increased risk of venous thrombosis (that is, having a vein clogged by a blood clot). The implant contains only one hormone, a progestogen called etonogestrel, which does not increase the risk of venous thrombosis in studies conducted outside the postpartum period. In our 2015 study, we found that the implant does not increase blood clotting compared to no hormone at all. In the 2019 study, we found that placing the implant within the first 48 hours after delivery did not change weight loss, and that the bleeding pattern in the first year after delivery is comparable to that expected when the implant is placed after the traditional 6-week waiting period after delivery. In short, we found that the implant does not affect women’s health if placed shortly after delivery.
Given these indications, could the implant constitute an alternative for family planning? What are the advantages and disadvantages of this method?
Carolina Sales: The World Health Organization has actually considered implants as an option for postpartum family planning since they were first introduced in 2002, and since 2015 has recognized that implant placement is safe for women and their baby alike even within the first 48 hours of delivery, before hospital discharge. Our studies played an essential role in getting this method cleared for use sooner than 4 weeks after delivery.
The advantages of this method are its long duration of effect—at least 3 years; its high efficiency, with a 1-year failure rate of 5 per 10,000 users (10 times more effective than tubal ligation); it does not increase the risk of venous thrombosis, as there is no estrogen in its composition; it can be used by women with most health problems, as it does not affect blood pressure or diabetes, among other conditions; it reduces menstrual cramps; and it may improve premenstrual tension.
When considering disadvantages, it is important to note that this contraceptive is not associated with any serious side effects, unlike estrogen-containing contraceptives. Some women do experience irregular bleeding during the first months of use, which can be bothersome if the patient has not received proper guidance, but has no negative impact on health. It is also worth noting that 80% of women are satisfied with their bleeding pattern. Another side effect that may be bothersome is acne (pimples or spots), which affects one out of every ten women who get an implant, but is treatable.
Do you see potential for this implant as a public policy for Brazil? What would it represent in terms of safety and effectiveness when compared to the most commonly used methods?
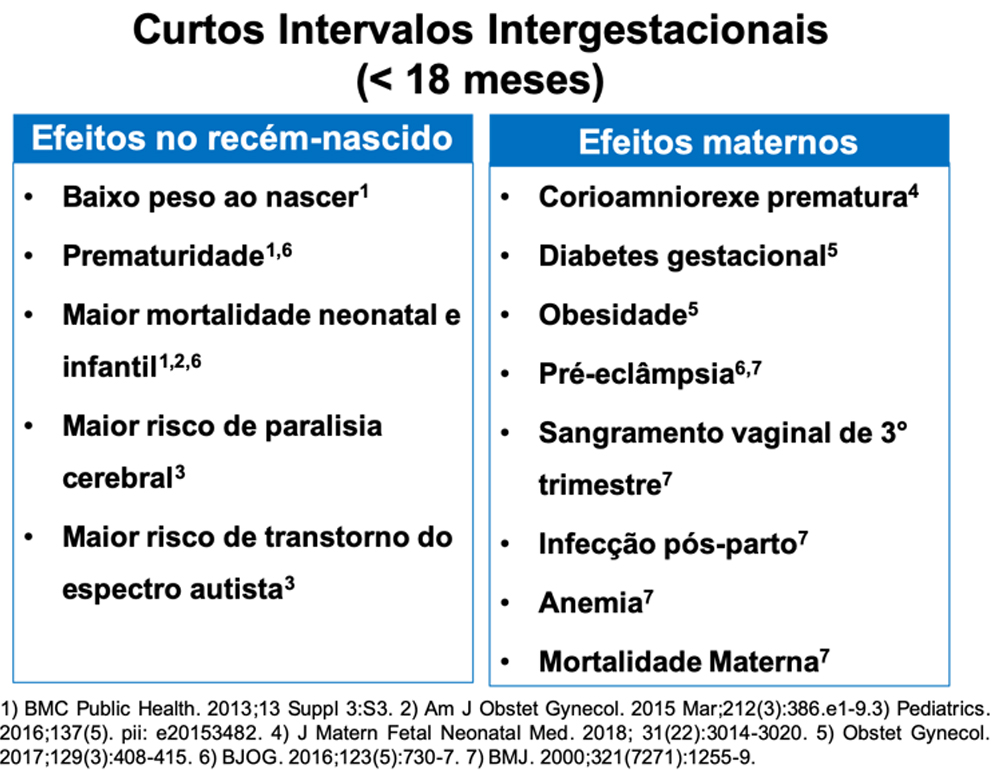
Carolina Sales: This practice is indeed recommended as public policy to reduce the rate of short interpregnancy intervals—that is, pregnancies which occur in rapid succession. A short interpregnancy interval, which is defined as less than 18 months between delivery and a new positive pregnancy test, increase the risk of poor health outcomes both for women and for their children (see Figure below). Often, taking advantage of the event of childbirth to start a contraceptive method is very convenient for women. This is because up to 40% of women fail to attend their 40-day postpartum visit, at which time a contraceptive would traditionally be prescribed; these women are thus at risk of unplanned pregnancy. According to U.S. studies, for every 1,000 women who get the implant immediately after delivery, more than 1 million dollars are saved over 3 years and 191 additional pregnancies are prevented as compared to waiting 6 weeks after delivery. Again, this difference is because some women do not attend their postpartum visit and end up having an unplanned pregnancy.
Currently, we tend to use more short-acting methods, such as the pill and injectable contraceptive. The effectiveness of these methods depends on women remembering to take them. The implant and intrauterine devices (IUDs) are considered long-acting methods, and their effectiveness doesn’t on rely on your memory. Accordingly, studies show that the pill is 21 times more likely to fail than implants or IUDs.
What about the cost of eventually incorporating this method into the Brazilian Unified Health System? Do we have any data on the cost-effectiveness of subcutaneous contraceptives? Are further studies needed?
Carolina Sales: Currently, the Unified Health System only offers one reversible long-acting contraceptive: the copper IUD, which actually could be used more widely than it is today; less than 2% of women have a copper IUD. However, the inclusion of other long-acting methods, such as contraceptive implants, has been considered cost-effective in all studies conducted to date in other countries. This is because the cost of carrying an unplanned pregnancy to term in Brazil is R$2,300 (approximately US$1,000 in 2014), which is much higher than the price of any contraceptive method. Up to 55% of pregnancies in Brazil are unplanned. Providing this method through the Unified Health System could help reduce this rate, as well as address other women’s health problems such as unsafe abortions and maternal mortality. One study found that including long-acting methods such as implants and IUDs was able to reduce abortions and teenage pregnancies by 50%. We can only hope that Brazil will start to invest on preventing unplanned pregnancies in a more evidence-based manner.
What is your advice for women who might be interested in this alternative?
Carolina Sales: Women should seek a health care facility and ask their provider about this method. Reliable websites can also be a good source of information. Some cities make this method available to special patient groups, such as adolescents, but it is generally not available through the Unified Health System.
Recommended further reading:
1) 2019 Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, eight change, 12-month continuation and satisfaction rates: a randomized controlled trial;
2) 2017 Timing of etonogestrel-releasing Implants and growth of breastfed infants: a randomized controlled trial;
3) 2015 Immediate postpartum initiation of etonogestrel-releasing implant: A randomized controlled trial on breastfeeding impact;
4) 2012 Effects of the etonogestrel-releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: a randomized controlled trial;
5) 2009 Safety of the etonogestrel-releasing implant during the immediate postpartum period: a pilot study.




